by Joseph A. Gallo, DSc, AT, PT, and Kevin J. Silva, MS, AT
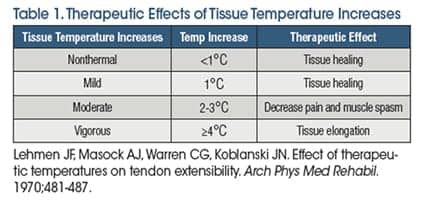
Superficial and deep heating agents have been widely used in rehabilitation to elevate tissue temperature to therapeutic levels. Superficial heating agents, such as hot packs, hot water immersion, and paraffin baths, are limited to 1-2 cm depth of penetration. In addition, superficial heating agents are not capable of producing the 3-4°C increases in intramuscular temperature needed for effective heat and stretch interventions.1 Shortwave diathermy and therapeutic ultrasound are classified as deep heating agents. A distinguishing feature of shortwave diathermy and therapeutic ultrasound is their ability to heat tissue to 3-4°C at depths of up to 5 cm.2,3 It has been made clear in the research literature that clinicians must be intentional with their use of heating agents in order to achieve the desired therapeutic outcome (Table 1).4 This includes considering the depth of the target tissue, the desired level of tissue temperature change, and physiological limitations of the selected thermal agent.
Therapeutic Heating
When the treatment goal is to increase tissue extensibility, the research shows that the target tissue must be heated to the therapeutic level described as vigorous heating. Vigorous heating is defined as increasing subcutaneous tissue temperature to ?4°C above baseline tissue temperature, which is approximately 36°C. Shortwave diathermy has been shown to be the most effective deep heating agent when targeting large areas of deep tissue.2
Shortwave Diathermy
The term “diathermy” is from the Greek derivative, meaning “to heat through” (dia = through; thermy = heat). The electromagnetic energy produced by a shortwave diathermy (SWD) device can elicit both thermal and nonthermal physiologic effects in human tissue. Shortwave diathermy produces electromagnetic energy at 27.12 million cycles per second (MHz). Both pulsed shortwave diathermy (PSWD) and continuous shortwave diathermy (CSWD) have been shown to be highly effective and efficient in increasing tissue temperatures to therapeutic level (?4.0°C) at depths of 3-5 cm.2,5,6 Conversely, superficial heating agents are unable to produce comparable therapeutic heating levels, and do not promote the same gains in tissue extensibility as compared to shortwave diathermy or thermal ultrasound.6 Shortwave diathermy is best suited for deep heating of larger areas, while thermal therapeutic ultrasound is best suited for heating areas that are two to three times the area of the sound head.
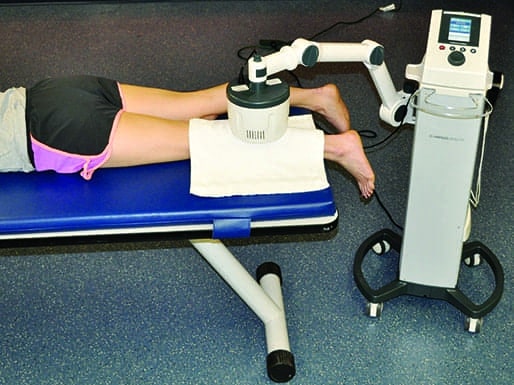
Shortwave diathermy can be delivered using either a capacitive applicator or an inductive applicator. Both application techniques result in energy conversion of the electromagnetic field to a mechanical energy that creates physiologic effects within the tissue (Figure 1). This occurs in the patient’s tissue through the stimulation of ion acceleration (molecular vibration), which produces either a thermal or nonthermal effect depending on the selected dose. The capacitive technique delivers SWD between two plates comprised of a rigid metal or a flexible rubber material, depending on the manufacturer. The capacitive applicators are used to bracket the target tissue. The energy is absorbed mostly in superficial tissues with high electrical impedance such as skin and fat, and has been associated with a higher risk for burns. The capacitive technique is seldom used in the United States. Consequently, this feature will focus exclusively on the inductive field technique.
Inductive Field Technique
Inductive field SWD generators release energy from a coil housed within a drum (monode). The energy preferentially heats low impedance tissues (ie, skeletal muscle, blood, and synovial fluid). The inductive technique is the most common applicator because it is capable of heating deeper tissue to moderate to vigorous heating levels. The drum is attached to a pivoting arm that extends off of the SWD device. It is recommended that a towel be draped over the area being treated prior to positioning the drum.
Shortwave Diathermy Compared to Therapeutic Ultrasound for Deep Heating
Decreased muscle flexibility and joint hypomobility are two common impairments that rehabilitation professionals treat. When selecting a deep-heating agent prior to stretching or joint-mobilization techniques, many clinicians turn to therapeutic ultrasound. Therapeutic ultrasound parameters can be manipulated to heat deep muscle; however, ultrasound is an inefficient heater of deep muscle and is unable to heat larger areas effectively. Muscle absorbs less ultrasound energy than more collagen-rich tissue. Therefore, longer treatment times are necessary to increase tissue temperature to desired therapeutic levels.7 For example, at 1 MHz and 1.5 W/cm2 it would take up to 14 minutes to increase deep tissue temperature to vigorous levels (?4.0°C).
Shortwave diathermy matches the depth of penetration and heating rate of 1 MHz ultrasound, with the added benefit of heating a larger area without the need for manual application by the clinician.1 In addition, soft tissue treated with SWD holds tissue temperature changes three times longer than an ultrasound treatment.8 Muscle maintains its peak temperature for 3.3 minutes post-ultrasound treatment, while SWD extends this window to 10 minutes.8 Likewise, tendons and ligaments maintain therapeutic heating levels for up to 5 minutes post-ultrasound treatment, which is extended to 15 minutes following SWD treatment.8 SWD extends the stretching window providing more time for passive stretching, joint mobilization, and soft tissue mobilization before the tissue temperature retards to a baseline level.2,8,9 During and immediately after SWD treatment, stretching techniques can be performed to effectively promote tissue elongation.
Pulsed Shortwave Diathermy
Draper and his colleagues2,3,6,8-12 have led the way in SWD research in the United States. Their work focuses on using pulsed shortwave diathermy (PSWD) as a deep heating agent. It often seems counterintuitive to clinicians that PSWD can heat, but it’s clear from the work of Draper et al. that it can heat efficiently and, when used in combination with a stretching regimen, can improve flexibility in subjects with tight hamstrings and plantar flexors.9,11 In 2014, Draper found that a combination of PSWD and joint mobilization was an effective therapeutic intervention in postoperative elbow patients with decreased joint range of motion. These patients had not responded to previous interventions to restore range of motion. However, the combined intervention was able to increase active elbow extension up to 25 degrees within four to six visits.12
Continuous Shortwave Diathermy
More recent research has shed light on continuous shortwave diathermy and stretching as a combined treatment intervention. In 60 subjects with impaired hamstring flexibility, continuous shortwave diathermy at 50 Watts for 20 minutes followed by hamstring stretching (three static bouts held for 30 seconds each) resulted in increased flexibility compared to a stretching-only group. The group that receive both CSWD and stretching showed an increase in hamstring flexibility of 13.70 compared to 8.10 in the stretching-only group. Additionally, the CSWD group and stretching group showed greater sustained hamstring flexibility 24 hours post-therapeutic interventions compared to the stretching-only group.13 In a more recent study that replicated the previously mentioned methodology, 39 subjects with hamstring tightness received a combination of CSWD and stretching that produced an increase in hamstring flexibility (13.70) compared to the stretching-only group (7.00). This study also concluded that the combination of CSWD and stretching showed greater sustained hamstring flexibility up to 48 hours post-intervention.14
Dosing Shortwave Diathermy
The research provides guidance on dosing thermal shortwave diathermy. However, additional considerations to dosing SWD must be taken in patient populations that have impaired sensation and/or circulation. These individuals may not be able to dissipate heat as well as healthy subjects used in research studies. It is also likely that the heating rates may vary between SWD manufacturers and devices. It is important to note that dosing the intensity of SWD is based on patient feedback and tolerance. The clinician must also carefully screen patients for contraindications to SWD, and follow appropriate safety precautions which are widely available in therapeutic modality textbooks and manufacturer operator manuals.
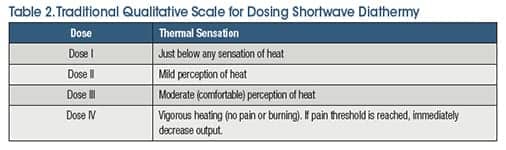
The patient’s perception of thermal sensation is an important factor when dosing SWD. In addition to the specific parameters provided by research studies, traditionally a four-level dosing model (Table 2) that focuses on patient perception of thermal sensation has been widely accepted. Newer technology has expanded the traditional four-level dosing model to a six-level model, to achieve greater control of therapeutic dosage levels allowing for safer and more efficient heating.
The SWD parameters most often associated with vigorous heating (4°C increase from baseline) are based on the work of Draper et al. using PSWD for 15 to 20 minutes (pulse width of 400 microseconds and a pulse rate of 800 pps).1 CSWD research on the dose response relationship associated with heating and stretching has established quantitative parameters supporting the use of 50 Watts for 20 minutes with stretching during and immediately post-treatment.13
Conclusion
Both PSWD and CSWD can serve as an efficient, safe deep-heating agent that can enhance the effectiveness of passive stretching, joint mobilization, or soft-tissue manipulation. RM
Joseph A. Gallo, DSc, AT, PT, is a professor and director of the athletic training program at Salem State University, Salem, Mass.
Kevin J. Silva, MS, AT, is the program assistant and adjunct faculty for the athletic training program at Salem State University, Salem, Mass. For more information, contact [email protected].
References
1. Ostrowski J. Comparison of muscle temperature increases produced by moist hot pack and thermoStim probe. J Sport Rehabil. 2018;6:1-20.
2. Draper DO, Knight K, Fujiwara T, Castel JC. Temperature change in human muscle during and after pulsed short-wave diathermy. J Orthop Sports Phys Ther. 1999;29(1):13-22.
3. Garrett CL, Draper DO, Knight KL. Heat distribution in the lower leg from pulsed short-wave diathermy and ultrasound treatments. J Ath Train. 2000;35(1):50-55.
4. Lehmann JF, Masock AJ, Warren CG, Koblanski JN. Effect of therapeutic temperatures on tendon extensibility. Arch Phys Med Rehabil. 1970;51(8):481-487.
5. Goats, GC. Continuous short-wave (radio-frequency) diathermy. Br J Sports Med. 1989;23(2):123-127.
6. Draper DO, Hawkes AR, Johnson AW, Diede MT, Rigby JH. Muscle heating with megapulse II shortwave diathermy and rebound diathermy. J Athl Train. 2013;48(4):477-482.
7. Watson T. Ultrasound in contemporary physiotherapy practice. Ultrasonics. 2008;48(4):321-329.
8. Draper DO, Knight KL. Therapeutic Modalities: The Art and Science. 2nd Ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013.
9. Peres SE, Draper DO, Knight KL, Ricard MD. Pused shortwave-diathermy and proglonged long-duration stretching increase dorsiflexion range of motion more than identical stretching without diathermy. J Ath Train. 2002;37(1):43-50.
10. Draper DO, Harris ST, Schulthies SS, Durrant E, Knight KL, Ricard M. Hotpack and 1 MHz ultrasound treatments have an additive effect on muscle temperature increase. J Ath Train. 1998;33(1):21-24.
11. Draper DO, Castro JL, Feland B, Schulthies S, Eggett S. Shortwave diathermy and prolonged stretching increase hamstring flexibility more than prolonged stretching alone. J Orthop Sports Phys Ther. 2004;34(1):13-20.
12. Draper DO. Pulsed shortwave diathermy and joint mobilizations for achieving normal elbow range of motion after injury or surgery with implanted metal: A case series. J Ath Train. 2014;49(6):851-855.
13. Holcomb KE, Scifers JR, McNamara SE. The effects of CSWD on hamstring flexibility. MAATA Annual Meeting, 2015.
14. Holcomb KE, Grimsley EE, Scifers JR. Carry-over effects of CSWD and stretching in developing hamstring flexibility. North Carolina Athletic Trainers’ Association Annual Meeting, 2016.