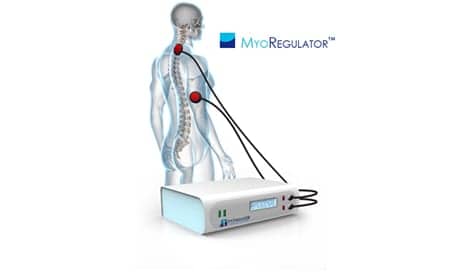
PathMaker Neurosystems Inc, developer of the MyoRegulator for the treatment of spasticity, announces it has launched an Institutional Review Board (IRB)-approved human clinical trial of the product.
The trial is being performed in conjunction with Northwell Health and The Feinstein Institute for Medical Research to evaluate the MyoRegulator’s safety and efficacy.
“I see patients who can no longer open doors or feed themselves as a result of suffering from spasticity,” says Bruce T. Volpe, MD, the study’s lead investigator and an investigator of the Laboratory of Biomedical Science at the Feinstein Institute, in a media release.
“It is my hope that at the conclusion of this trial, we will see that MyoRegulator is a safe and effective treatment option,” he adds.
PathMaker also announces that the MyoRegulator study has received non-significant risk (NSR) designation from the FDA. This designation allows clinical trials to proceed in the US without the need to submit an Investigational Device Exemption (IDE) application for approval, because the trials, as assessed by the FDA, are not considered to present a potential for serious risk to the health, safety, or welfare of a subject.
The MyoRegulator was also one of the first products designated for FDA’s Expedited Access Pathway (EAP), a new FDA pathway intended for breakthrough products to facilitate patients gaining more rapid access to critical medical devices by expediting their development, assessment, and review, according to the release.
The MyoRegulator device features DoubleStim technology for multi-site neurostimulation. According to the release, animal studies suggest that the DoubleStim technology may be able to suppress the hyperexcitable spinal circuits that result from spasticity.
[Source(s): PathMaker Neurosystems Inc, Business Wire]




I am suffering from spasticity for over 4 years and would like to know more about this study and be part of this work
I also would like to know more about the trial for this product. I live in the United States.
I would like to know what are the requirements to get enrolled in the myo regulator trial