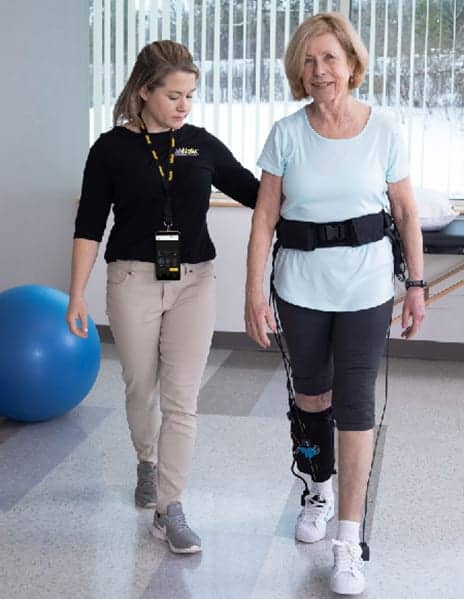
The US Food and Drug Administration (FDA) has cleared ReWalk Robotics Ltd’s ReStore soft exo-suit system for sale to rehabilitation centers across the United States, the company announces in a media release.
ReStore is intended for use in the treatment of stroke survivors with gait and mobility challenges resulting from stroke.
“The exo-suit achieves our commercial goal to offer a functional and affordable system that can be utilized in the ‘Main Street’ clinics in every community,” says ReWalk CEO Larry Jasinski.
“Soft Suit” Design
The ReStore system is comprised of a soft, garment-like design which connects to a lightweight waist pack and mechanical cables that help lift the patient’s affected leg in synchronized timing with their natural walking pattern. The system provides targeted assistance to the patient during forward propulsion (plantarflexion) and ground clearance (dorsiflexion), two key phases of the gait cycle.
What’s In It for Therapists
The device also provides physical therapists with extensive data during gait training with ReStore to inform strategies to optimize a patient’s treatment and progress using real-time analytics, the release explains.
“The idea of anchoring the body with textiles and flexible soft components is a fundamentally new way of applying assistance with a wearable robot,” comments Conor Walsh, a professor of engineering and applied sciences at the John A. Paulson Harvard School of Engineering and Applied Sciences and a Core Faculty member at the Wyss Institute for Biologically Inspired Engineering
Walsh led a multi-disciplinary team to develop the soft exo-suit technology that has the ability to apply force to the body, but not restrict how a person moves.
Therapy and Mobility Possibilities
“This technology has broad potential, and we are currently testing additional concepts which can be applied to provide therapy and/ or mobility assistance for individuals with other diseases, such as multiple sclerosis and Parkinson’s disease, and also potentially be used by a person at home and in their community,” Walsh continues.
ReStore is ReWalk’s second major market segment, joining the ReWalk Personal 6.0—a robotic exoskeleton for home use by individuals with paralysis from a spinal cord injury—and the ReWalk Rehabilitation system, which allows for multiple patient uses through height and weight adjustments.
[Source(s): ReWalk Robotics Ltd, PR Newswire]